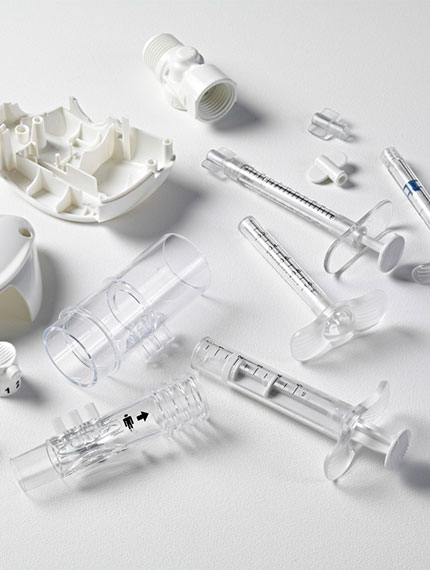
At the core of the operations of Plas-Tech Engineering lies Plastics and Injection Molding. We have built a reputation over 30 years as the expert in plastic component manufacturing, where plastic plays a crucial role.
Our commitment to serving medical device manufacturers includes significant investment in cleanroom technology and processes that meet USP Class 6, 7, and/or 8 standards for product manufacturing. Our team of engineers possesses extensive knowledge in materials and practices essential for producing components and filtration accessories in these controlled environments.
Our Cleanroom Injection Molding Includes:
- Glass Alternative or Replacement Molding (available in COC material)
- In-Mold Labeling and In-Mold Decoration (IML/IMD)
- ISO Class VI (1000) Cleanroom Post Processing
- Micro-Molding
- Multi-Cavity Collapsible Core or Expandable Core Molds
- Multi-Cavity Unscrewing Molds
- Multi-Shot or Two-Shot Molding
- Multi-Material and Multi-Component Molding
- Overmolding
- Insert Molding
Other Key Differentiations are:
- Glass Alternative or Glass Replacement Molding
- USP Class VI and ISO 10993 Materials
- Sterilizable Materials for Gamma, ETO, or Steam Autoclave
- Modern, High-Precision Electric Injection Molding Machines
- Thin Wall Molding (as low as .006)
- ERP Software and Warehouse Management Integrated with the Machines
- Press-Side Assembly, Sub-Assembly, Pad Printing, Hot Stamping, and Ultrasonic Welding