
PTE Adds New Cleanrooms and Services to Production Floor
Plas-Tech Engineering announced the addition of a new cleanroom designated for specialty assembly and parts cleaning. This new cleanroom enhances the current Class 6 operations with the ability to ultrasonically …
Learn More
Plas-Tech Adds New CNC Lathe Tuning Equipment to the Toolroom!
PTE is pleased to announce the addition of a new CNC lathe turning equipment. “This new capability will increase our speed and throughput within our tooling operation,” says President/CTO of …
Learn More
Plas-Tech Engineering Adds a New High-Speed CNC Machine to its Toolroom
PTE has the intent of being as vertically integrated as possible. To accomplish this, we have added a brand-new high-speed CNC machine to our toolroom! This is the second CNC …
Learn More
Plas-Tech Supports FDA on UDI with Collaboration with Matrix IT
Announcing the inclusion of Unique Device Identifiers in EHR Certification Criteria and the Common Clinical Data Set This week marked an important milestone for the adoption of unique device identifiers …
Learn More
Plas-Tech Engineering Announces a NEW Tamper-Evident Syringe Case for Pre-Fillable Syringes
This new and novel design allow pharma companies to pre-fill and store syringes in a travel-style and tamper-evident case. The case is gamma/EtO sterilizable and has additional options for size …
Learn More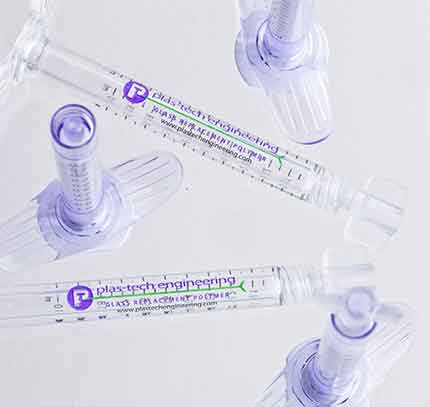
Plas-Tech Engineering launches Glass-Replacement PFS Syringe Line!
This PFS glass-replacement syringe line is designed specifically for light-sensitive drug formulations. It is the first-ever of its kind.
Learn More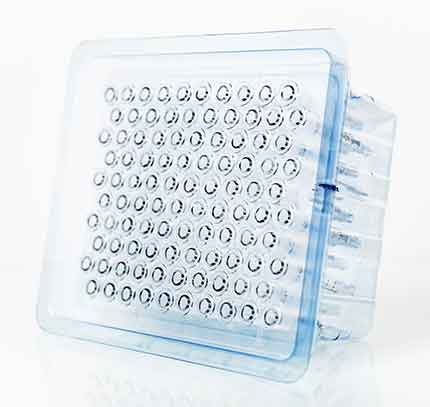
Plas-Tech launches new Equinox brand SmarTUB® Nested Syringes for the Prefilled Syringe (PFS) market!
These syringes are a direct fit for automated filling lines requiring nested tubs. The designs meet ISO 11040 standards and can be completely customized with your choice of polymers (COC-Topas, …
Learn More